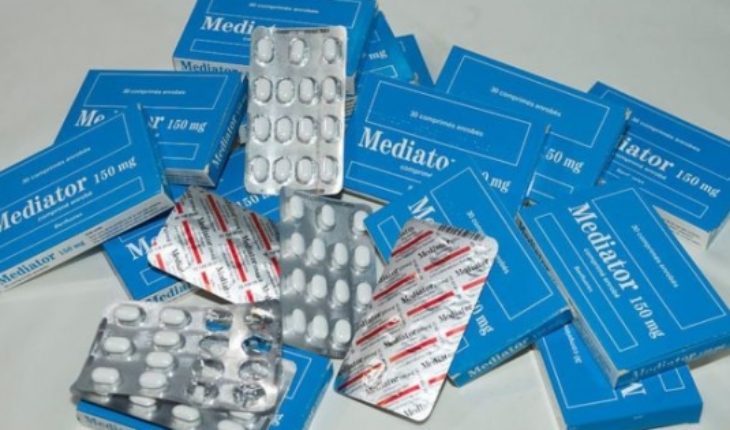
Servier Laboratories, one of France’s largest pharmaceutical companies, and health authorities in that country will have to prove this week that they were not aware of the lethal risks of Mediator, a drug that was sold during decades as an appetite suppressant and could have caused nearly 2,000 deaths, according to judicial experts.
The scandal erupted a decade ago, but the alleged victims have had to wait until this Monday for the start of a mega-trial in which there will be thousands of participants and that will last until April 2020.
On one side are the prosecution and more than 2,500 particular accusations. On the other, Servier and the National Medicines Safety Agency along with nine other legal and 12 natural persons, including employees of the pharmacist and a former senator.
The process also comes five years after the death of the company’s president and founder, Jacques Servier, who was one of the main defendants, and that the firm, who claims he was not aware of the risks of the drug, paid more than 3,000 Compensation.
Jacques Servier, the founder of the company, was one of the main defendants but died five years ago. The offences charged include deception, fraud, influence and injury trafficking and homicide.
The company not only plays million-dollar compensation, but also runs the risk of being disabled for the exercise of its activity, according to the French press.
Diabetic medication
Mediator was the trade name for benfluorex and appeared on the market in 1976 as pills to treat overweight in patients with diabetes.
However, it soon began to be marketed as an appetite suppressant for those who wanted to lose weight.
Benfluorex increases the sensitivity of cells to insulin, so the body uses it better and reduces blood glucose levels, the European Medicines Agency (EMS) explained in a 2010 report.
In addition, it acts on the liver by increasing the production of glycogen (the way glucose is stored in the liver) and thus reducing the feeling of hunger.
Laboratorios Servier is one of the leading pharmaceutical companies in France.Mediator was prescribed to millions of people in France and other european countries for decades. Even in 2003, when it ceased to be sold in Spain and Italy, France continued to allow doctors to prescribe it and even subsidize it.
It was Iréne Frachon, a pneumologist, who had the drug removed from the market in 2009, two years after it first alerted health authorities to the potential harmful effects of Mediator.
Frachon had noticed that patients treated with Mediator had suffered from valvulopathies, diseases affecting the heart valves. Some died and others were left with aftermath and disabilities.
It was the pneumologist Iréne Frachon who got the authorities to withdraw Mediator from the market. After conducting a study that confirmed their fears, it was not until 2009 that what is now one of the biggest scandals that the French pharmaceutical sector has had to do.
More risks than benefits
The following year, the National Medicines Agency admitted that Mediator was linked to about 500 deaths.
In the same year, the AEM concluded that the effect of benfluorex on the treatment of diabetes was “limited” and that its benefits were not “greater than its risks”, and therefore recommended that, after 33 years of marketing, the drug be taken off the market.
The pharmacist admits on its website that “the adverse effects associated with the consumption of Mediator have had undeniably serious consequences for some patients and their loved ones”.
But she also criticizes the fact that many “unacceptable” statements have been made about her and calls for a “fair trial.”
Meanwhile, many wonder how a drug could have been prescribed for so long without anyone noticing that it was harmful.
translated from Spanish: The slimming pill scandal that France accuses of causing nearly 2,000 deaths
September 23, 2019 |