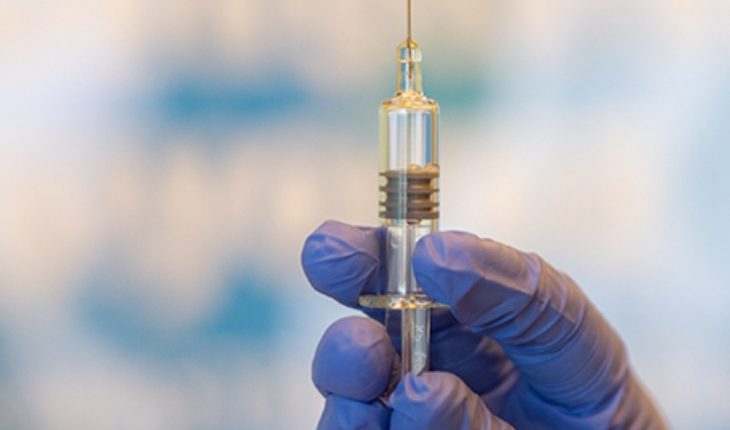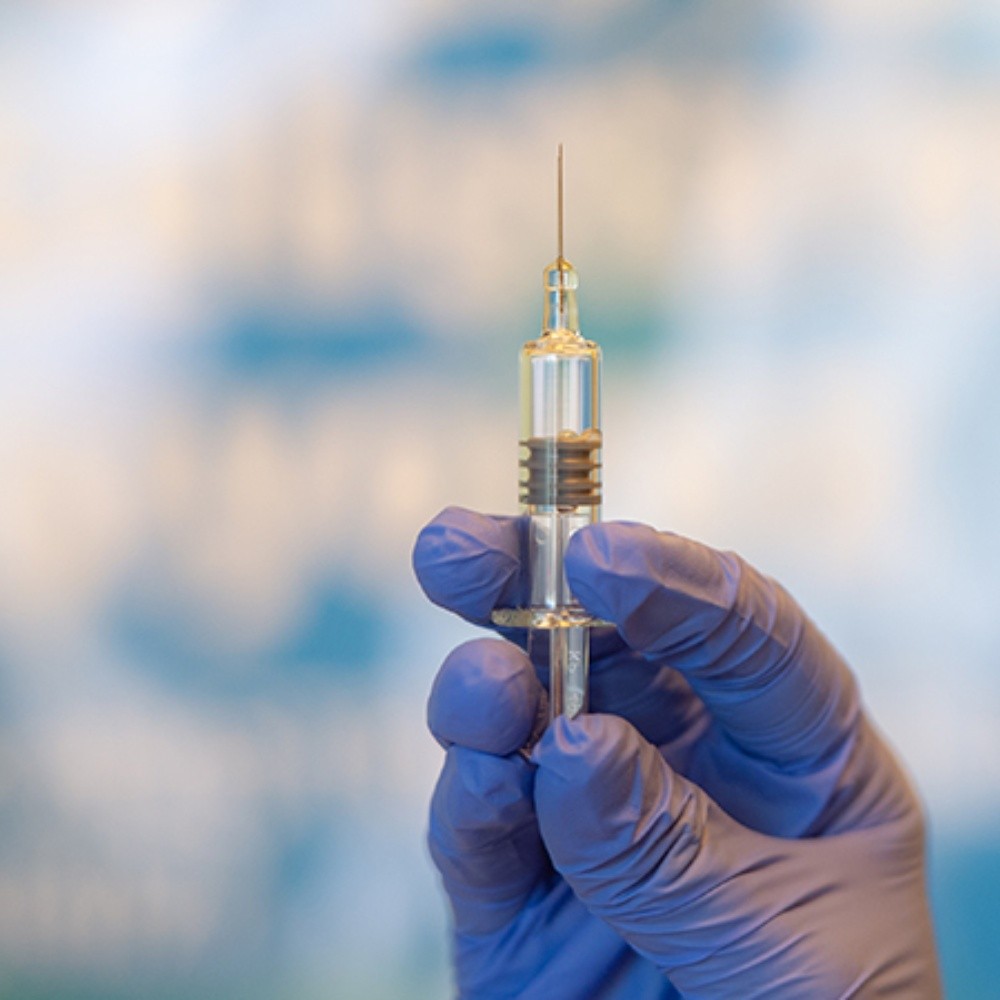
U.S.- Pfizer has released images of a possible coronavirus vaccine coming off the production line, as the pharmaceutical giant aims to have 100 million doses ready by the end of the year in the U.S. Unidos.La a Manhattan-based company said Sunday that it has already done “several hundred thousand doses” of the drug as it prepares to seek emergency use in the United States by November.
Stay informed about what matters most to you
Get the most relevant news of the day in your e-mail
Thank you for subscribing!
Check your inbox to confirm your email and start getting the latest news
Take advantage and take the next step
Get our news alerts so you don’t miss anything
Receive notifications
Not bad! You’ve subscribed to notifications
Set up and choose your preferences
Set up notifications
Enter your e-mail
Subscribe
Subscribing involves accepting the terms and conditions
Not bad! You’ve subscribed to notifications
Set up and choose your preferences
The company’s head in the UK, Ben Osborn, also told the newspaper that scientists at its main British laboratory have also discovered drugs that could provide a possible complete cure for Covid-19, rather than a simple preventive vaccine.
Osborn said the company is manufacturing the huge reserve of its current candidate vaccine in Belgium “at risk and on a large scale,” calling it “tremendously exciting.”
Pfizer is one of the leading companies in the development of the Covid-19 vaccine. Afp
“The hope here is that we will essentially come up with a drug that will disrupt the virus and ultimately prevent a patient’s condition from worsening,” he told the Mail’s financial site, This is Money.
It was great to see the first vial of the manufacturing line come out. It brought me a tremendous smile to see that all this work resulted in a product, he said.
But “we can only go as fast as science allows us,” he emphasized.
Pfizer hopes to know whether or not the vaccine is working by the end of this month, but the company has yet to prove that the injection is safe and that it can be properly manufactured to apply for so-called emergency use authorization, Executive Chairman Albert Bourla.Si said Friday, expects his company to seek emergency use in the U.S. In the third week of November. “We’re operating at the speed of science,” he said.