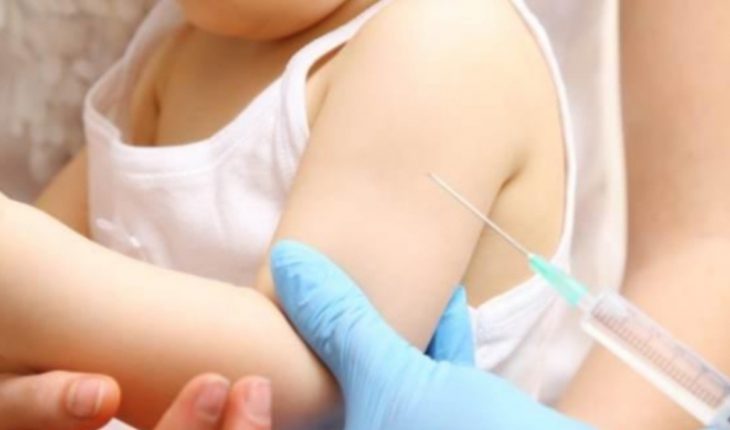
U.S. pharmaceutical company Pfizer announced Thursday that it has initiated clinical trials of its coronavirus vaccine in children 6 months to 11 years old. The first participants of the clinical trial have already received the initial injection of this vaccine, which Pfizer developed together with the German laboratory BioNTech.For the study, Pfizer joins Moderna who is also conducting studies to reach children in this age range.
For this initial phase, scientists aim to have 144 children with which they want to identify the best dose for three age groups: between 6 months and 2 years, between 2 and 5 years and between 5 and 11 years.
Children will start receiving a dose of 10 micrograms of the serum to increase it progressively, according to Pfizer, although participants also have the option to choose the dose of 3 micrograms, compared to the two doses of 30 micrograms that adults receive. After the doses are applied, they will go to the second stage where the effectiveness and safety of the doses applied will be analyzed, as well as the adverse effects they may cause. Let’s remember that in this class of trials, of the 144 participants some receive the dose of the medication and some simply receive a placebo. The pharmacist announced in a statement that : “Pfizer has a lot of experience in conducting clinical trials of vaccines in children and infants and is committed to improving the health and well-being of children in these trials that are designed with consideration.” Pandemic specialists also say this step is critical to controlling the pest and the progression and spread of the virus.
Inoculation of children, who account for about 20% of the US population, is essential to end the coronavirus pandemic, noting that the country is unlikely to achieve collective immunity until children are also vaccinated.
Pfizer’s announcement comes two days after the pharmacist claimed to have initiated the early stages of a clinical trial of an oral drug that could be used after the first symptoms of covid infection. In the statement further explained that its drug, called PF-07321332, has been shown in in vitro studies to be a “powerful protease inhibitor with antiviral activity against SARS-CoV-2” and other coronaviruses, suggesting its “potential” for the treatment of covid-19 and other “threats”. However, Johnson & Johnson also plans to test his single-dose vaccine on newborn infants and infants after doing so in older children.