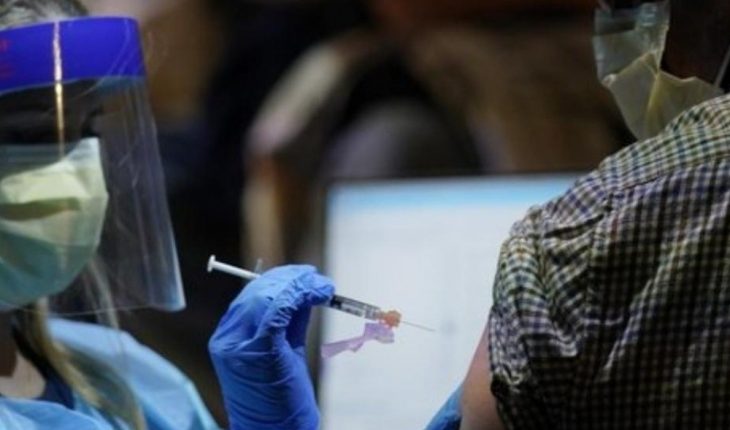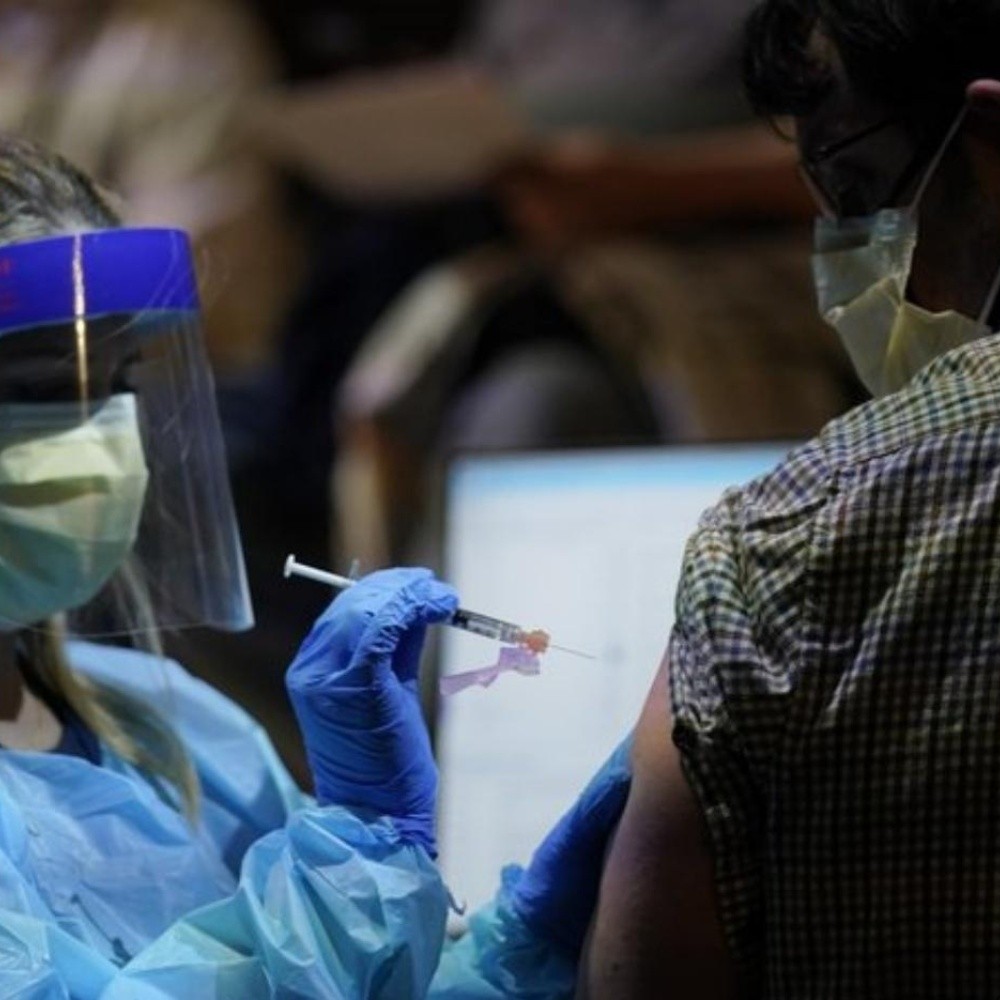
United States.- On Thursday the U.S. Food and Drug Administration (FDA), authorized the administration of a third dose of the Pfizer and Moderna vaccines against Covid-19 for people who are immunocompromised or who have received an organ transplant or some other condition in order to protect them from more aggressive variants of Covid-19.This after the FDA will conduct a thorough review of the Pfizer and Moderna vaccines to ensure viability of a third application to certain population groups.” After a thorough review of the available data, the FDA determined that this small and vulnerable group may benefit from a third dose of the Pfizer or Moderna vaccines,” the Agency’s acting commissioner, Janet Woodcock, said in a statement. With this decision, the Food and Drug Administration will apply to millions of citizens in a vulnerable situation to suffer from the disease due to transplants of orranos or certain types of conditions such as cancer, in a similar way to countries such as France and Germany who have also opted for the immunocompromised to receive a third dose of the vaccine. The authorities have stressed that the decision only applies to this group of people and ruled out that it is an opportunity to receive a third booster dose to the general population. Read more: Historic number of migrants arrested at Mexico – USA Border Although the United States has done a monumental job in its vaccination process by inoculating authorities’ concerns have re-emerged after the number of new infections rose to more than 100 thousand cases a day in recent weeks, a figure not seen since January 2021. The Bejuco River overflows in Nayarit and floods Mexico 15
translated from Spanish: U.S. approves third dose of Covid-19 vaccine
August 13, 2021 |