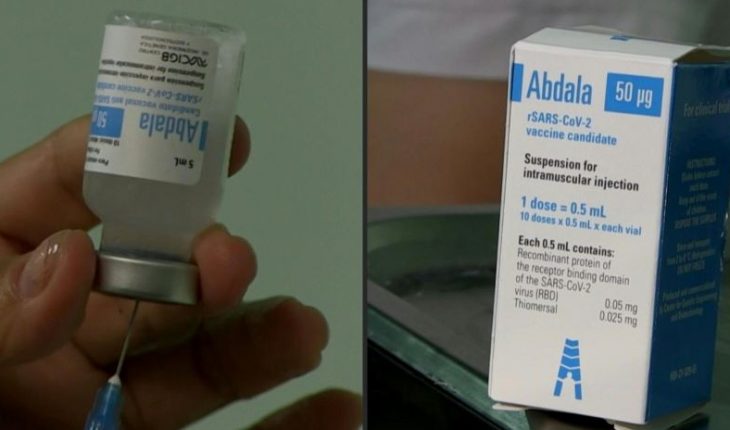
The Federal Commission for the Protection against Sanitary Risks (Cofepris) issued a favorable opinion for the emergency use of the Abdala vaccine, to prevent COVID-19.
This is the first vaccine of Latin American origin to be analyzed by the Commission’s New Cells Committee, and it represents a step prior to the authorization of emergency use.
“The biological is made with the recombinant protein of the receptor binding domain of the SARS-CoV-2 virus,” Cofepris explained in a statement, so the next step for its approval will be the submission of the pharmaceutical company’s file.
Committee on New Molecules? issues favorable technical opinion for emergency use of the COVID-19 vaccine Abdala.?
? https://t.co/0sOHAJmb7z pic.twitter.com/YTfxSBZipL
— COFEPRIS (@COFEPRIS) August 31, 2021
Abdala, along with Soberana 02 and Soberana Plus, is one of Cuba’s vaccines that have been used to immunize 3.17 million people in that country.
Cuba is the only Latin American country that has its own vaccines, however they have not been recognized by the WHO, until now.
In Mexico there are nine vaccines approved by authorities against COVID: Pfizer-BioNTech, AstraZeneca, CanSino Biologics, Sputnik V, Sinovac, Covaxin, Janssen and Moderna, and Sinopharm, authorized only on August 26.
What we do at Animal Político requires professional journalists, teamwork, dialogue with readers and something very important: independence. You can help us keep going. Be part of the team.
Subscribe to Animal Político, receive benefits and support free journalism.#YoSoyAnimal