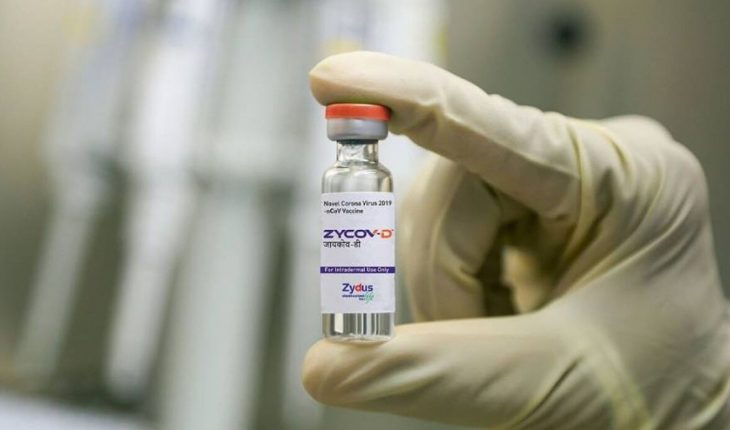
India recently approved a new vaccine that uses circular strands of DNA to prepare the immune system against the SARS-CoV-2 virus and researchers, who celebrate the news because it “shows that we have another class of vaccines that we can use,” say many other DNA vaccines could soon “nip at your heels.” Named ZyCoV-D, it is administered to the skin without an injection and has 67% protection against symptomatic Covid-19 in clinical trials; starting to be administered in India probably this month.
About a dozen DNA vaccines against the novel coronavirus are in clinical trials globally, and a few others are in earlier stages of development, Nature reports. DNA vaccines are also being developed for many other diseases. Among the benefits are detailed, on the one hand, that they are easy to manufacture; and on the other, that finished products are more stable than RNA vaccines, which generally require storage at very low temperatures. In this case, ZyCoV-D, which was developed by the Indian pharmaceutical company Zydus Cadila and whose clinical trials involved more than 28,000 participants, was also authorized for people from the age of 12. As for its mechanism, ZyCoV-D contains circular strands of DNA known as plasmids that encode the SARS-CoV-2 spike protein along with a “promoter” sequence to activate the gene. Once the plasmids enter the nuclei of human cells, they become mRNA, and once in the main body of the cell, the cytoplasm, is translated into the spike protein itself. The body’s immune system then generates a response against the protein and produces adapted immune cells that can eliminate future infections. Plasmids usually degrade in weeks or months, but immunity remains.
In pink, the nucleus of cells; in blue, the cytoplasm.
The novelty, the scientists argue, has to do with the challenge of DNA vaccines: they need to reach the cell nucleus, unlike mRNA vaccines, which only need to reach the cytoplasm. That’s why, unlike conventional vaccines that are applied to muscle tissue known as deltoids, ZyCoV-D is administered using a needle-free device that is pressed against the skin, in an area underneath it that captures DNA much more efficiently. However, for this it requires a minimum of three doses. On the other hand, regarding the 67% efficacy that in principle may seem low compared to those currently approved, they highlight that the ZyCoV-D trials in India earlier this year were carried out while the Delta variant was predominantly circulating, while the RNA vaccine trials were conducted when less transmissible variants were circulating, so that would be another piece of good news. Meanwhile, Zydus Cadila is still expected to publish the results of the last stage of the trial still underway, the full analysis of which, the company says, will be shortly.