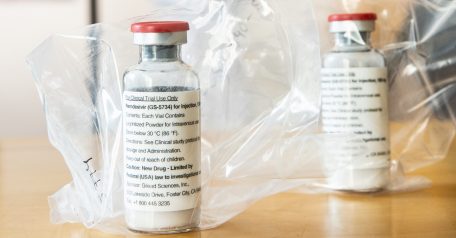
The European Commission provisionally authorised the use of the antiviral medicine remdesivir on Friday for the treatment of patients with coronavirus in the European Union (EU), following the approval of the European Medicines Agency (EMA).
The Community executive granted “a conditional marketing authorisation for the remdesivir medicinal product, making it the first authorised eu-wide remedy for the treatment of COVID-19,” he announced in a statement.
“We grant this authorization less than a month after the application was filed,” said Health Commissioner Stella Kyriakides, for whom this demonstrates “the EU’s determination to respond quickly” to new treatments.
Read: Treatment with remdesivir reduces recovery time of COVID-19 patients, research reports
Conditional marketing authorisation is reserved in the EU for drugs, whose benefits are estimated to be greater than their risks, despite not yet having complete data. Its duration is one year renewable.
The molecule from the US laboratory Gilead Sciences was developed to treat patients with Ebola hemorrhagic fever, without success, but during a us trial it demonstrated some efficacy against COVID-19.
According to this study, patients with this disease and treated with remdesivir are restored about four days before the other patients. Its use has already been authorized in cases of emergency in the United States and Japan.
Find out: Treatment with remdesivir was effective in Mexicans critical by COVID-19, says Salud
In its recommendation, prior to the Commission’s approval with the approval of the 27 European countries, the EMA proposes its use for adults and adolescents from the age of 12 years old suffering from pneumonia and in need of oxygen.
The new coronavirus has caused more than half a million deaths worldwide since it appeared in December, according to a balance from the AFP based on official sources. Europe is the deadest region in the world, nearly 200,000.
In parallel, scientists around the world are in a race to find a coronavirus vaccine.
What we do in Animal Político requires professional journalists, teamwork, dialogue with readers and something very important: independence. You can help us keep going. Be part of the team.
Subscribe to Animal Politics, receive benefits and support free journalism #YoSoyAnimal.
"El reclamo puede ser genuino, pero construido sobre una mentira", apuntó el presidente Javier Milei…
El gobernador de la provincia de Buenos Aires, Axel Kicillof, encabezó un acto en Ensenada…
El diputado nacional de La Libertad Avanza, José Luis Espert, expresó su confianza en la…
Tras la masiva reaparición de Cristina Fernández de Kirchner, el presidente Javier Milei apuntó contra…
El principal propósito de la nueva comisión es evaluar los recursos humanos en el Senado,…
En una medida que busca redefinir las condiciones de los seguros de automóviles en Argentina,…
Esta web usa cookies.