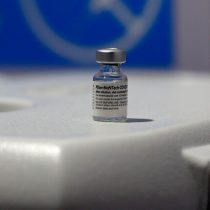
The U.S. Food and Drug Administration (FDA) granted its full approval to pfizer/BioNTech’s covid-19 vaccine, the first serum of its kind in the country to get a full green light from the regulator, as it has so far had only “emergency.
This was reported by the FDA in a statement, where its acting commissioner, Janet Woodcock, stressed that this is a milestone in the fight against the pandemic. Following this approval, “the public can be very confident that this vaccine meets the highest standards of safety, effectiveness and manufacturing quality that the FDA requires for a (fully) approved product,” Woodcock said.
The head of the FDA was hopeful that with the full approval will overcome the distrust of some people towards serums against covid-19. “Today’s milestone brings us one step closer to changing the course of the pandemic,” Woodcock said.
The vaccine was granted emergency use authorization in the U.S. in mid-December for those over the age of 16, and was expanded in May for those over the age of 12. Full approval applies only to those over the age of 16, as Pfizer/BioNTech, which from now on will be called Comirnaty, has to provide more data on the use of the vaccine in children between the ages of 12 and 15 to achieve full authorization, which could take months.
Emergency authorizations can be used by the FDA during health emergencies to provide access to medical products that can be effective in preventing, diagnosing, or treating a disease. Unlike an approval for emergency use, full authorization lasts indefinitely unless some kind of unexpected side effect develops. To achieve this type of approval, the company must provide expanded data on the manufacturing process and is subject to thorough FDA inspections.
The FDA is reviewing the information provided by Moderna, the other pharmaceutical company that has developed a messenger-RNA vaccine, such as Pfizer’s, to obtain full approval, as it only has the emergency use vaccine. The decision could take weeks. While in the case of Johnson&Johnson, it is expected to file its application for full authorization soon.
The FDA is also now reviewing the possibility of giving a booster dose of pfizer and moderna vaccines to people already immunized, which the government wants to start administering from September 20 in the face of the spread of the delta variant.
"El reclamo puede ser genuino, pero construido sobre una mentira", apuntó el presidente Javier Milei…
El gobernador de la provincia de Buenos Aires, Axel Kicillof, encabezó un acto en Ensenada…
El diputado nacional de La Libertad Avanza, José Luis Espert, expresó su confianza en la…
Tras la masiva reaparición de Cristina Fernández de Kirchner, el presidente Javier Milei apuntó contra…
El principal propósito de la nueva comisión es evaluar los recursos humanos en el Senado,…
En una medida que busca redefinir las condiciones de los seguros de automóviles en Argentina,…
Esta web usa cookies.