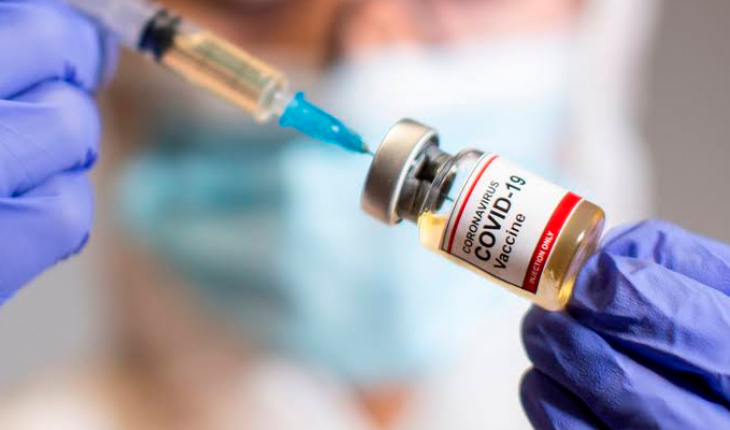
Michoacán.- This Friday as part of phase 3 studies of the COVID-19 vaccine, Chinese-Canadian doses developed by CanSino Biologics will begin to be applied in Mexico City, and 5,000 healthy volunteers are intended to participate.
In videoconference with the head of government, Claudia Sheinbaum, the researcher Guillermo Ruiz-Palacios, of the National Institute of Medical Sciences and Nutrition “Salvador Zubirán” (INCMNSZ) explained that the start of this clinical trial will be a “slow” process, which will start with five volunteers over 18 years of age who meet the necessary requirements to receive the biological.
People with diabetes or hypertension, but controlled, may participate, and people who have already had COVID-19 are discarded.
He also explained that volunteers will be recruited at INCMNSZ, as well as in three clinics of the Secretariat of Health (Sedesa) of the capital government: “José Castro Villagrana”, in Coyoacán, as well as the “David Fragoso Lizalde” and the “Pedregal de las Aguilas”, both in the Tlalpan mayoral.
In addition to receiving the application of the biological, people who decide to participate in the trial will be under medical supervision for up to two years and will be guaranteed care for any reactions derived from the test.
He detailed that the protocol begins with blood sampling and a nasopharyngeal exudate, to rule out that they are not infected with COVID-19. The volunteers are then vaccinated and monitored for any “respiratory events”. At 28 days they are taken a new blood sample to know the antibody response.
Participation in this protocol will have no payment. Volunteers will only receive financial transportation support to the medical center where the dose is applied.
Von the start of phase 3 of the CanSino vaccine, Mexico City joins the states of Guerrero and Oaxaca, where it already applies. A total of 16,000 people are expected to participate in different entities; in Michoacán they would start next week, although the protocol is still unknown by health authorities, as these are private laboratories.
The Chinese-Canadian vaccine, developed by CanSino Biologics and named Ad5-nCOV, caused a strong antibody reaction in another trial in most of the approximately 500 participants.
The CanSino vaccine is based on a modified adenavirus that does not replicate, making them safer, especially for the most fragile patients
None of the tests generated any serious undesirable effects. The most commonly observed side effects were fever, fatigue, and pain at the injection site of the vaccine.
translated from Spanish: Today starts COVID-19 vaccine test on CDMX, next week in Michoacán
November 13, 2020 |